Products
BioEngine has developed nearly 100 types of serum-free media for different cell lines, which are suitable for antibodies, vaccines and cell and gene therapy (CGT) fields.
BioEngine is not only a media products supplier, but also a solution provider for biopharmaceuticals, vaccine production, and cell and gene therapy fields. Based on a large amount of historical data, powerful analytical tools and extensive project experiences, BioEngine can advise you on culture model decisions, process robustness control, product quality optimization, and bioreactor operation enhancement, and ultimately help you achieve or enhance your existing process at commercial scale, creating higher value.
It is suitable for the development and optimization of customized serum-free/protein-free medium for large-scale, high-density, suspension culture processes of various animal cells. This technology has been successfully applied to the pilot development and industrialization of many Chinese antibody drug and virus vaccine manufacturers. The specific cell growth rate, maximum viable cell density, antibody concentration and virus titer are higher than those of global serum-free media.

This technology regulates the physiological state and metabolic network of cells by designing and optimizing the nutrient composition of the medium and process parameters of fed-batch or perfusion strategies, thus improving nutrient metabolism and energy utilization. Compared to the batch process or the previous process, the peak viable cell densities increase significantly, the cell viabilities maintained a high level for a longer time, the product yields improved, and simultaneously, the costs significantly decreased.

This technology aims to solve the problems of easy breakage of animal cells culturing in bioreactors and the difficulties on industrial scale-up, and to meet the requirements of process intensification for high-density culture. Through extensive and in-depth scientific research, BioEngine has established powerful technologies for cell culture process and bioreactor scaling-up and enhanced operation, which can effectively prevent the breakage of animal cells. BioEngine has the ability to design and develop bioreactor systems, laying a solid foundation for industrialized-scale, high-density cell culture processes.

Guided by the Quality by Design (QbD) concept, R&D team of BioEngine made a great effort to analyze the product expression processes at the bioreactor level, cellular level and molecular level, and understand the relationships between protein qualities and process parameters, so as to establish high-yield, high-quality and stable animal cell culture process through rational design and optimization of culture medium and process parameters.

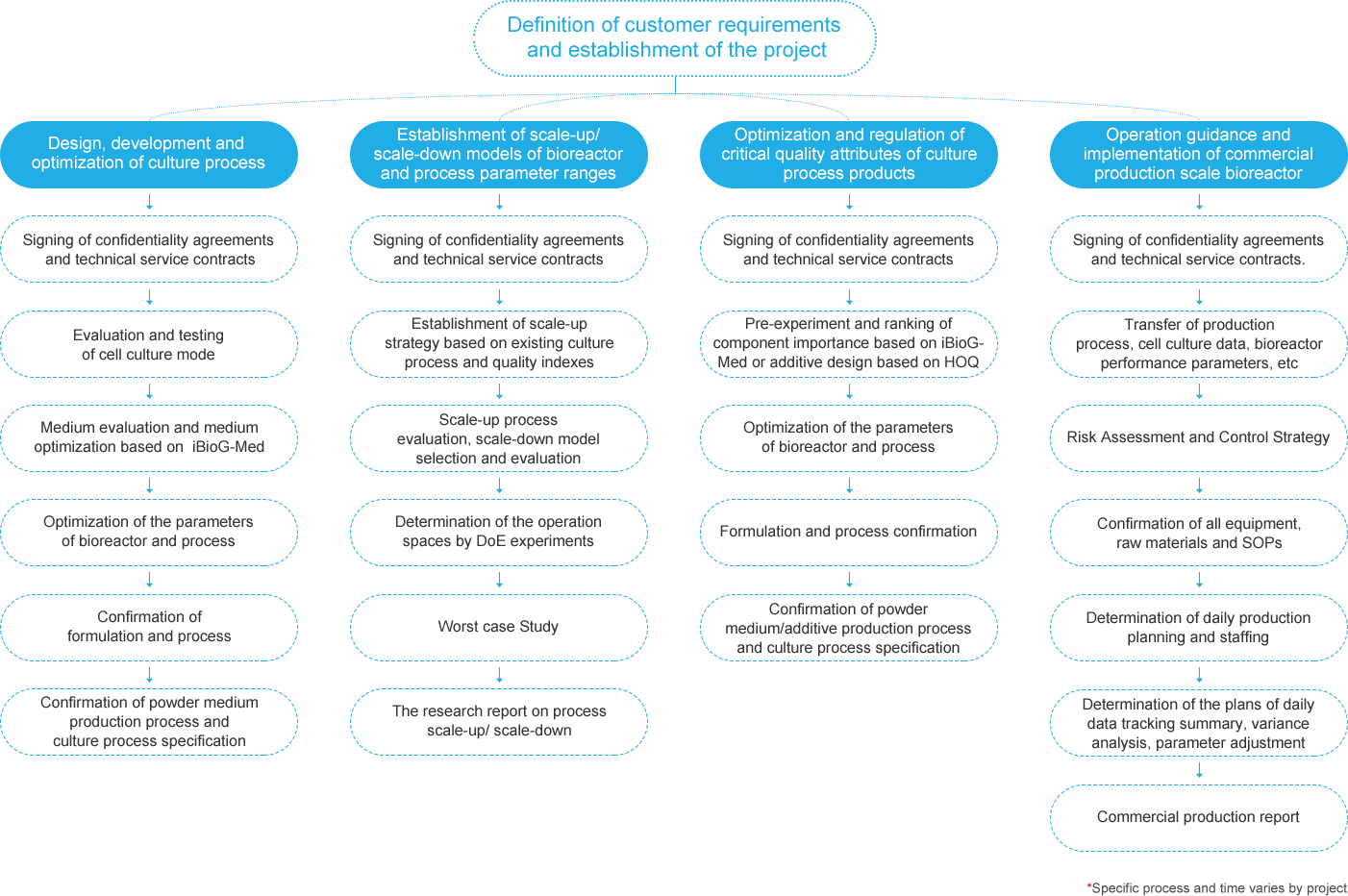
With four major technology platforms, massive historical data, powerful analysis tools and rich project experiences, BioEngine can provide you with quality services and create higher value.
We have a professional technical team to provide high quality one-stop cell culture service from process development
and optimization, medium formulation design to medium processing and manufacturing for all biopharmaceutical companies.
If you have any question, please click "Quick Message" and leave your message, we will reply to you as soon as possible.
If anything urgent, please call (86)21-68582660-2792.
![]() 3F&4F, Building 3, Lane 396, Lvzhou Ring Road, Minhang District, Shanghai, PRC
3F&4F, Building 3, Lane 396, Lvzhou Ring Road, Minhang District, Shanghai, PRC