Products
BioEngine has developed nearly 100 types of serum-free media for different cell lines, which are suitable for antibodies, vaccines and cell and gene therapy (CGT) fields.
Customized media development services can help increase titer, improve protein quality, and reduce drug discovery and commercial production costs.
With strong R&D capabilities, a large library of in-house media formulations and a high-throughput screening-based iBioG-Med serum-free media development platform, BioEngine can provide a series of customized services, such as medium formulation development for specific projects, process enhancement for fine-tuning and controlling of critical quality attributes (glycoform, charge heterogeneity, acidic peaks, fragmentation, sialic acid, etc.) of drugs, and production of customized media. BioEngine can not only quickly obtain high-performance medium formulation, which can increase the efficiency by 30-50% compared with traditional strategies, but also accurately identify key components and quickly determine the effective concentration range, providing scientific basis and guidance for quality control of subsequent commercial production of dry powder medium.
30 years of experience in cell culture technologies
Large library of cell culture media formulations
In-house iBioG-Med platform for medium development
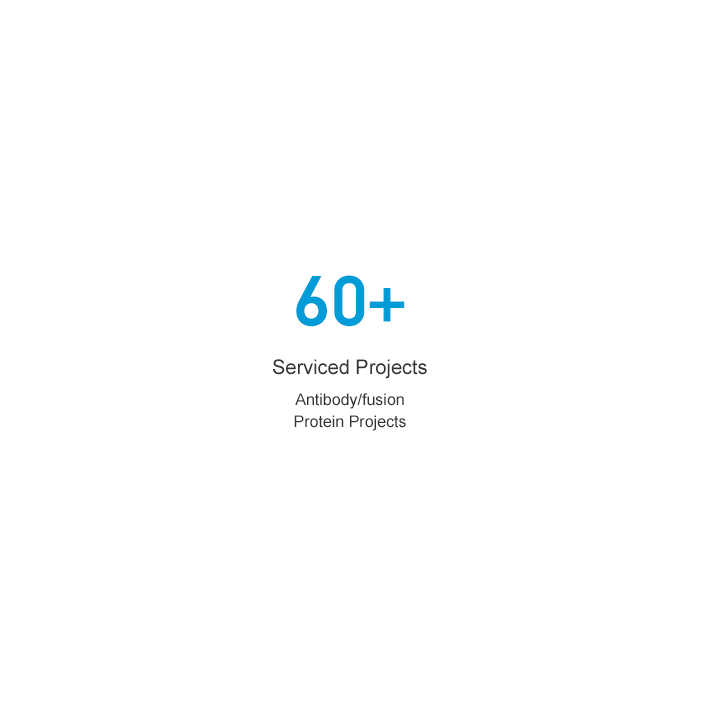
Abundant project experiences
60+ customized development projects, 30+ clinical projects
Customization of platform culture medium for platform cell lines
Customization for specific projects
Adjustment of medium components to optimize the ratio of glycoform
Adjustment of medium components to optimize charge heterogeneity
Adjustment of medium components and process parameters to optimize the ratio of acidic peaks
Adjustment of medium components to optimize the level of high-mannose glycoforms
Adjustment of medium components and process parameters to optimize fragmentation levels of monoclonal antibody
Adjustment of media components and process parameters to optimize the sialic acid levels of fusion proteins
Other custom development services and optimization projects

Biosimilars and innovative drugs (Monoclonal antibody/multi-specific antibody/Fc fusion protein)
Traditional targets (CD20/HER2/PD1 etc.) and new targets (CD38/IL6 etc.)
We have a professional technical team to provide high quality one-stop cell culture service from process development
and optimization, medium formulation design to medium processing and manufacturing for all biopharmaceutical companies.
If you have any question, please click "Quick Message" and leave your message, we will reply to you as soon as possible.
If anything urgent, please call (86)21-68582660-2792.
![]() 3F&4F, Building 3, Lane 396, Lvzhou Ring Road, Minhang District, Shanghai, PRC
3F&4F, Building 3, Lane 396, Lvzhou Ring Road, Minhang District, Shanghai, PRC