Products
BioEngine has developed nearly 100 types of serum-free media for different cell lines, which are suitable for antibodies, vaccines and cell and gene therapy (CGT) fields.
Time:Jan 17,2023
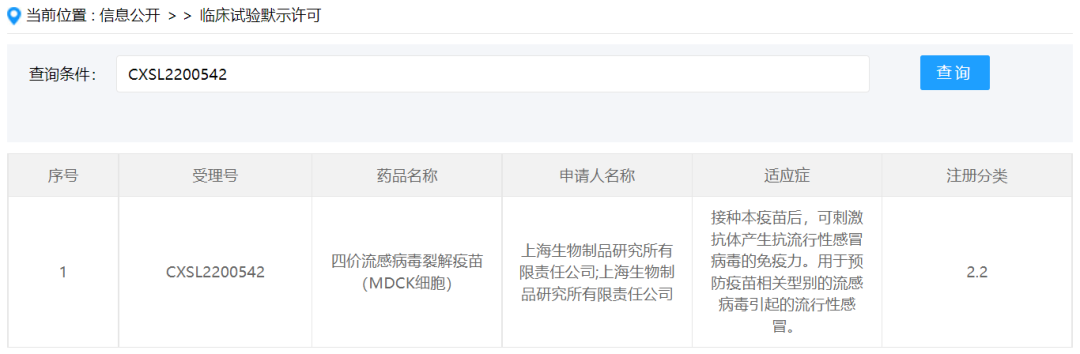
On January 13, the quadrivalent influenza split-virion vaccine (MDCK cells) independently developed by Shanghai Institute of Biological Products Co., Ltd. was issued a Notice of Approval for Drug Clinical Trials by the National Medical Products Administration. This vaccine is an important scientific achievement in the field of influenza vaccine development and is also the first influenza vaccine based on serum-free whole suspension cell culture approved to enter clinical trials in China. Currently, there is no influenza vaccine product based on a cell culture platform available in China, and cell serum-free suspension culture is more suitable for scaling up production. The approval of the influenza vaccine from the Shanghai Institute of Biological Products Co., Ltd. for clinical use is an important technological innovation and breakthrough.
Although the development of novel influenza vaccines has become relatively simple based on decades of influenza virus research, the most widely used vaccine production process is still using chicken embryo culture. Chicken embryos have disadvantages such as unstable supply of raw materials and long production cycle. Vaccine production based on the chicken embryo method would be difficult to produce billions of doses of vaccine in a short period of time. Cell culture production systems are capable of producing large quantities of vaccines in a short period of time and can significantly improve the ability of people to cope with epidemics and emerging viral diseases. Therefore, the use of animal cell culture instead of the chicken embryo method for influenza vaccine production has gained increasing interest and application.
MDCK cell lines can efficiently support the infection and proliferation of human or avian influenza viruses and are widely used in influenza virus isolation as well as vaccine research and production. Moreover, MDCK cells are easily adapted to the serum-free culture and domesticated suspension culture and are more suitable for large-scale production by large bioreactors. The Xeno series of MDCK serum-free medium products of BioEngine can support the high-density suspension culture of MDCK cells without serum, which is suitable for the efficient expansion and production of all types of influenza viruses.

BioEngine is committed to the research and development of animal cell mass culture technology for a long time. Through continuous innovation, we now have animal cell serum-free culture products covering the whole line of vaccine products, which have been applied to more than 100 projects of human or animal vaccines. We have helped customers to improve virus yield, effectively reduce production costs and provide strong power for vaccine production.
We have a professional technical team to provide high quality one-stop cell culture service from process development
and optimization, medium formulation design to medium processing and manufacturing for all biopharmaceutical companies.
If you have any question, please click "Quick Message" and leave your message, we will reply to you as soon as possible.
If anything urgent, please call (86)21-68582660-2792.
![]() 3F&4F, Building 3, Lane 396, Lvzhou Ring Road, Minhang District, Shanghai, PRC
3F&4F, Building 3, Lane 396, Lvzhou Ring Road, Minhang District, Shanghai, PRC